Home Sensing Primer Glossary Physics Related Technologies Acknowledgements
Absorption spectra
Another type of spectrum is called an absorption spectrum. This spectrum is of supreme importance to those doing remote
sensing. Consider a beam of continuum radiation emitted by some glowing object (like our sun); suppose this beam strikes a solid surface
and is reflected. Some of the energy in the beam will be absorbed by the solid surface--the exact wavelengths absorbed and the degree of
absorption both depend upon the nature of the reflecting surface. A spectral analysis device would classify the reflected light as an
absorption spectrum.
As an elaborating example of the formation of absorption spectra, recall the beam of continuum radiation we talked about on the
previous page, i.e.
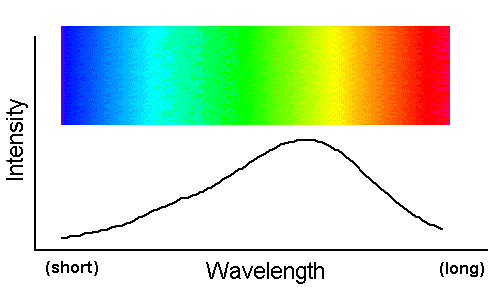
Suppose this beam struck a field of bright blue flowers. Suppose further that these flowers appear blue to our human eyes because they absorb yellow photons while reflecting all others (i.e. instead of calling them "blue," an organism with eyes more technologically sophisticated than ours might say the flowers actually appear "all colors except yellow"). The following spectral figure indicates the yellow photons that the blue (i.e. really "yellow-absorbing") flowers have absorbed from the incident light.
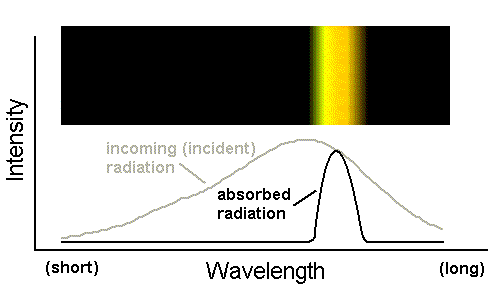
The spectrum above is not really anything that can be observed directly; it is just a representation of the energy removed from the beam. However, the energy reflected by the flower can certainly be observed. What does its spectrum look like? The figure below shows that it looks like a continuum spectrum, but with the yellow photons removed.
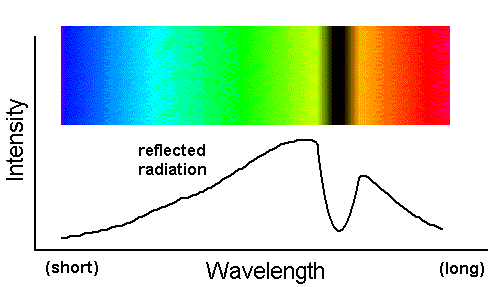
In this figure you can see the depression in the yellow part of this spectrum where the continuum energy was
sapped of the majority of its yellow photons. Hence, the flowers look blue. The dip in the yellow portion of this absorption spectrum
is called an absorption feature.
Since every kind of atom or molecule has its own unique combination of absorption features, it is theoretically possible
to use remote sensing of reflected spectra to determine the chemical composition of the surfaces of objects. For example, geologists use
remote sensing to classify exposed rock surfaces with great success. Their success is due in part because different minerals have very
different absorption features (different minerals can have extremely different chemical compositions).
Botanists have a much more challenging task because the characteristic colors in the leaves of most plants are due to the
same small set of pigments. As a result, the spectra of a dark green plant and a light green plant may differ only in the details.
Remote sensing practitioners are forced to try to classify vegetation using what at times are the most subtle of spectral differences.
To heighten the difficulty even more, factors such as plant maturity, illumination angles, nutrition status, season, etc., can create
spectral differences that overlap or even overwhelm the spectral differences between the various species being monitored.
Real spectra are much more complicated than my little cartoons, and are festooned with bumps, dips, valleys, and gorges.
Spectroscopists often refer to the spectral dips and wiggles due to (for example) a plant as a spectral signature. The goal is to recognize
some combination of features in the spectral signature that are unique to the target objects being mapped.
Note that while I have commented mostly on absorption features created when light reflects off an opaque object,
absorption features can also be created when light passes through transparent gases (such as our atmosphere), as we shall discuss more
fully in the next section.